Medical Devices
Compare-TAVI trial establishes non-inferiority of Myval THV series to Sapien3 THV series in a largest all-comer population
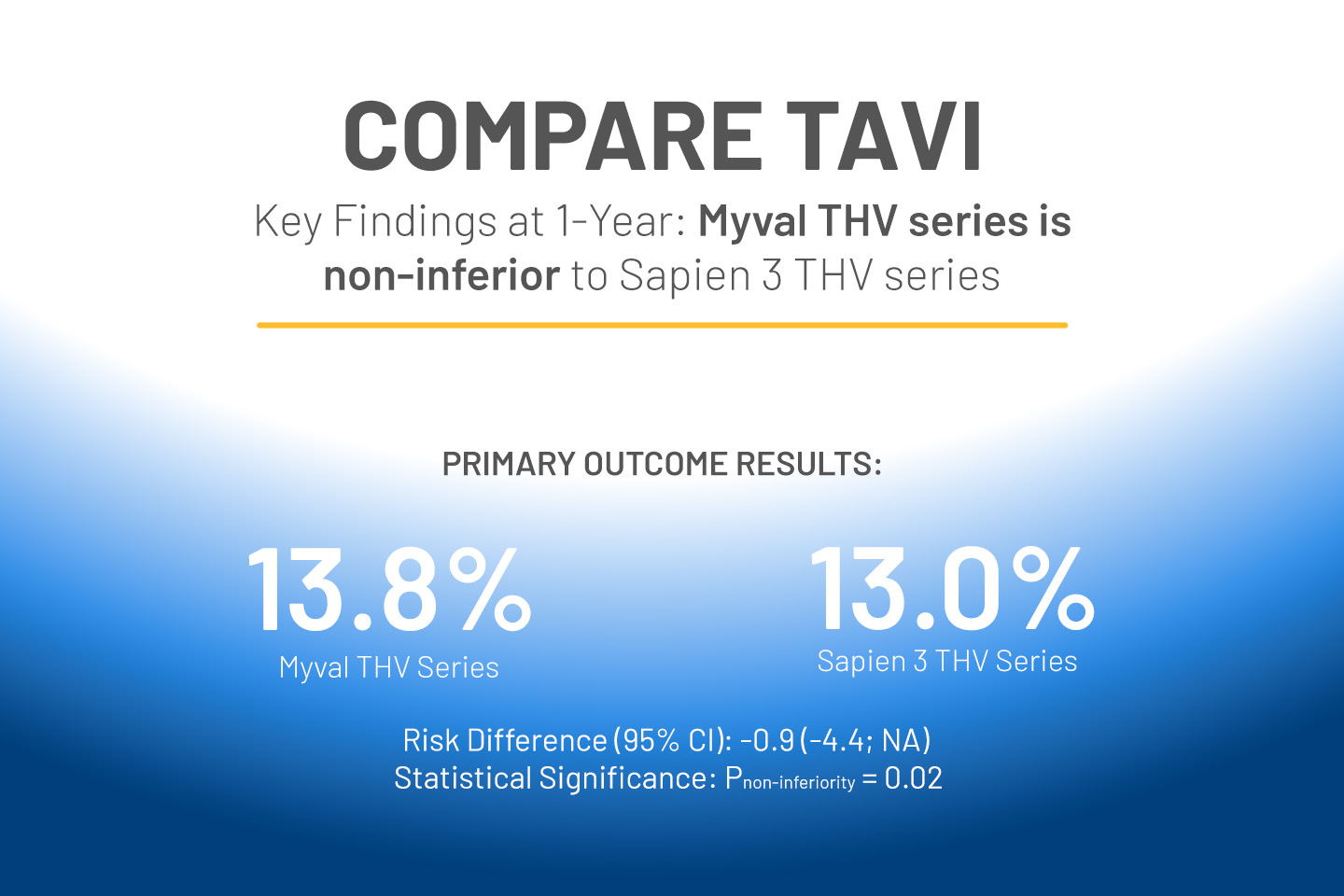
Meril Life is proud to announce the salient results of the Compare-TAVI trial, a landmark study comparing the safety and efficacy of transcatheter aortic valves — Meril’s Myval THV Series and Edward’s Sapien 3 THV Series. This is the largest randomized comparison of two balloon-expandable THVs in the all-comers population to date and the results are generalizable to daily clinical practice.
The trial was led by Professor Christian Juhl Terkelsen from Aarhus University Hospital Skejby, Denmark - who is the Principal Investigator, along with a distinguished team including Dr. Henrik Nissen who is the study chair from Odense University Hospital, Dr. Philip Freeman from Aalborg University Hospital, and Dr. Troels Thim from Aarhus University Hospital, Denmark.
Primary Endpoint at 1-year
The primary outcome results of the Compare-TAVI trial were presented under Late Breaking Trials by Dr. Henrik Nissen, at London Valves 2024, heralding a breakthrough. The Myval THV Series proved non-inferior to the Sapien 3 THV Series for primary composite safety and efficacy endpoint at 1-year follow-up (Myval THV series: 13.8% vs. Sapien 3 THV series: 13%; risk difference (95% CI): -0.9 (-4.4; NA); Pnon-inferiority=0.02). Also, the incidence of moderate or severe patient-prosthesis mismatch (PPM) was significantly lower in the Myval THV series than Sapien 3 THV series at 1-year [17.5% vs. 28.6%, P<0.001].
Study Design and Purpose
The Compare-TAVI trial is a real-world, randomized controlled trial with a 10-year follow-up aimed at comparing the outcomes of two balloon-expandable THV series: Myval THV series and Sapien 3 THV series. A total of 1,031 patients (Myval: 514 and Sapien 3: 517) were randomised in 1:1 ratio with a non-inferiority margin set at 5.3% at 80% power, with an assumed event rate of 13%. The primary endpoint at 1-year was a composite of death, stroke, moderate/severe aortic regurgitation (AR), and moderate/severe THV deteriorationaccording to VARC-3 criteria.
Adding more to the Future of TAVI: The Compare-TAVI trial is more than just a study; it is a pivotal milestone in interventional cardiology. With its rigorous design, cutting-edge imaging, and robust follow-up, this is the largest randomized comparison of two balloon-expandable TAVI valves in real-world populations. Meril Life Sciences is proud to contribute to this efficacious milestone in clinical research. As we acknowledge this achievement, we remain steadfast in our commitment to uplift global healthcare.
The Road Ahead: Looking ahead, the Compare-TAVI trial is pivotal for future advancements in TAVI technologies, ultimately improving patient outcomes worldwide. With ongoing follow-ups and analyses, Meril Life Sciences is poised to further solidify its role as a leader in innovation and patient-centered solutions.
Important Information:
| NCT04443023 | Randomized comparison of TAVI valves: The Compare-TAVI trial |
|
|
|







