Fighting the Good Fight: MedTech Innovations to Beat Coronary Artery Disease
Introduction
Heart disease—it’s a phrase that carries the weight of millions of lives. According to a report released by the British Heart Foundation earlier this year, around 620 million people are affected by heart and circulatory diseases worldwide. A significant contributor to this global health crisis is coronary artery disease (CAD), which accounted for 315 million cases in 2022 alone, driving both mortality and disability rates.
In India, the healthcare system faces an even steeper challenge, considering CAD-related mortality rates in the country are 20–50% higher than those in other populations. While prevention is still a cornerstone of heart health, there is an increasing need for medical interventions to manage this widespread condition.
In this context, innovations in medical technology play a crucial role in empowering healthcare professionals and improving patient outcomes. Cutting-edge advancements in MedTech devices can not only make procedures more efficient and minimally invasive, but also contribute to faster recovery times, reduced post-surgical complications, and ultimately, improved patient well-being. However, ensuring that these advancements remain affordable and accessible remains a critical priority.
At the heart of innovation: A closer look at Meril’s coronary portfolio
Meril’s indigenously developed coronary products empower healthcare providers to deliver the highest standard of care, thereby increasing the likelihood of positive patient outcomes. These devices are clinically backed by extensive studies involving patients from around the world, and supported by a team of global scientific advisors.
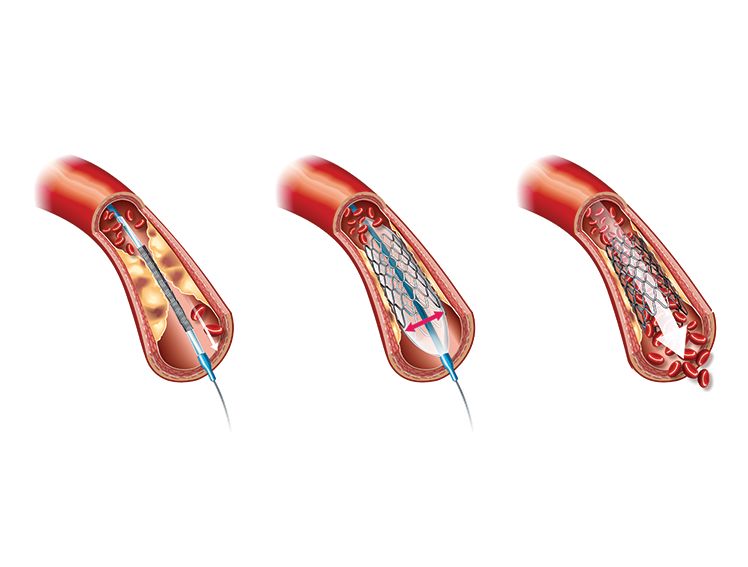
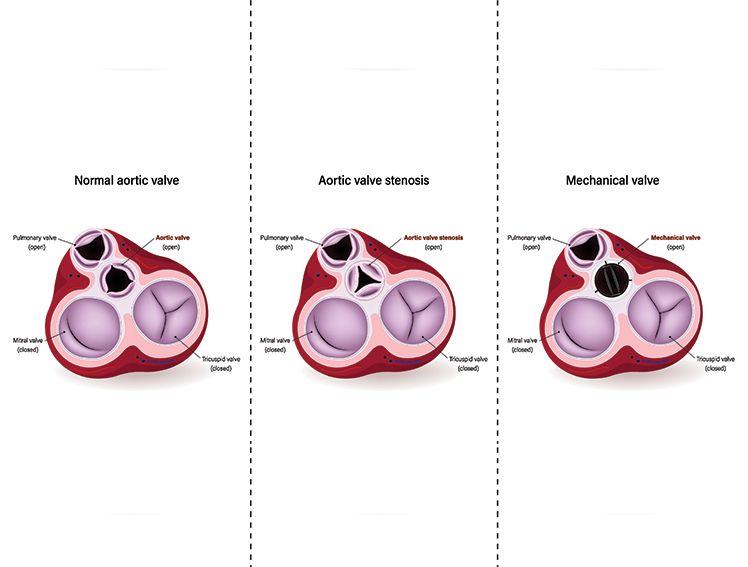
Percutaneous coronary intervention (PCI)—commonly known as Angioplasty, and an increasingly common treatment option in India, where the prevalence of CAD is 21.4% for diabetics and 11% for nondiabetics—involves the placement of stents, such as bare metal stents and drug-eluting stents (DES). DES are metal stents coated with a drug that prevents artery re-narrowing by limiting excessive tissue growth inside the stent post-angioplasty.
Meril’s BioMime™ DES is a Sirolimus Eluting Coronary Stent System that features a 65 µm strut. Other DES by Meril with the same strut thickness include the BioMime Morph™—a tapered Sirolimus Eluting Stent, the BioMime Lineage™, which is a Sirolimus Eluting Coronary Stent System on a Lineage Delivery System with Monolithic Design, and the BioMime Branch™, which is specially designed for bifurcations.
Meril’s latest breakthrough in DES technology, the EVERMINE50™, features an ultra-thin strut with a thickness of 50 μm, promoting early vascular healing. Both the BioMime series and the EVERMINE50™ also utilise a hybrid cell design, combining closed cells at the ends with open cells in the center to optimise side branch access and maintain radial strength for complex interventions.
Besides routine metal stents, bioresorbable scaffolds (BRS) have emerged as a promising alternative. Unlike conventional stents, which remain permanently in the artery, a BRS provides a temporary scaffold to the lesion, restoring blood flow while the artery heals. Once the vessel blockage is treated and healing is completed, the BRS dissolves fully, leaving the artery in its natural state with no foreign residue. This approach offers both physicians and patients greater flexibility for future treatment interventions, if needed, in the same blood vessel. BRS allow for successful acute revascularisation of coronary artery stenosis, and evidence shows that they have been associated with low rates of repeat angioplasty requirements and major adverse cardiac events (MACE) during the early follow-up period. Furthermore, the treated vessel may show a possible restoration in its vasomotor function when the structural integrity of the BRS has been appropriately lost.
Meril's MeRes100™ is a next-generation BRS that features a 100 µm-thin strut and sirolimus-eluting properties. The scaffold fully absorbs into the body within two to three years post-PCI, leaving behind a naturally functioning artery. Clinical studies have shown that patients treated with MeRes100™ experienced no scaffold thrombosis for up to three years, a significant milestone in the field of cardiovascular care. The MeRes100™ BRS has demonstrated positive long-term safety, with consistently low cardiac event rates and zero stent thrombosis in patients with coronary artery disease.
Meril’s portfolio also includes the MOZEC SEB drug-coated balloon (DCB) catheter, designed to treat in-stent restenosis by delivering sirolimus through a specialised nanotech drug delivery system—ensuring precise and targeted drug delivery to the affected area. This approach reduces the risk of re-narrowing and enhances long-term outcomes, making it a valuable tool in the management of complex coronary artery diseases, such as in-stent restenosis (ISR), small vessels, etc.
Beating the odds: Shaping a healthier tomorrow
For healthcare professionals, the reliability and advanced design of Meril’s coronary devices mean greater confidence in performing complex procedures, leading to more successful patient outcomes. For patients, these devices offer life-saving treatments that allow them the chance to live a longer life.
Meril's unwavering commitment to innovation is not just about developing new technologies; it's about transforming the future of cardiovascular care. By engaging in continuous research and fostering collaborations with leading medical professionals around the world, Meril continues to refine and advance the tools needed to tackle the pressing challenges of cardiovascular disease. This dedication ensures that patients and healthcare providers have access to the most effective and cutting-edge solutions, helping to improve heart health on a global scale. As Meril pushes the boundaries of what's possible, they are not only addressing the needs of today but also paving the way for the breakthroughs of tomorrow, ultimately shaping a healthier future for all.