MYOSIST
Intra Aortic Balloon Catheter Kit (8F)
- Myosist is an IAB catheter kit that is operated with an IAB pump.
- The device is placed in the descending aorta. By balloon inflation in the aorta during diastole and deflation during systole, it increases blood supply to the Heart muscle and decreases the workload of the left ventricle.
- During diastole, the balloon inflates and increases blood flow to the coronary arteries, increasing myocardial oxygen supply.
- During systole, the balloon deflates and this causes a decrease in pressure in the aorta; this decrease in pressure assists the left ventricle by reducing the pressure that needs to be generated to achieve ejection through the aortic valve.
Benefits
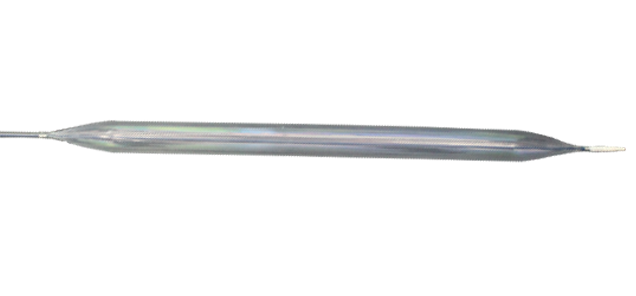
Highly Durable & Flexible Balloon Membrane Tested for 1 million inflation-deflation cycles for functionality and performance integrity.
Radiopaque Catheter Tip & Markers Easy to locate the MYOSIST deployment under fluroscopy. Catheter consists of optimized placement of radiopaque markers and radiopaque tip
Optimized Insertion Length With 710 mm insertion length, the MYOSIST is placed in proper positioning.
Inner Lumen Diameter 0.028 inch diameter provides a medium to measure excellent arterial pressure
Indication
Cardiogenic shock :
- Associated with acute MI, mechanical complications of MI - MR , VSD
In association with CABG :
- Preoperative insertion
- Patients with severe LV dysfunction
- Patients with intractable ischemic arrhythmias
- Postoperative insertion
- Postcardiotomy cardiogenic shock
In association with nonsurgical revascularization:
- Hemodynamically unstable infarct patients
- High risk coronary interventions
- severe LV dysfunction, LMCA, complex coronary artery disease
CVTS In Cardiopulmonary Bypass:
- Coronary bypass Graft
- Cardiac valve repair (Aortic, mitral, tricuspid, pulmonary)
- Repair of large septal defects (atrial septal defects, venticular septal defects)
- Transplantation (Heart and Lung).
*Disclaimer: Myosist IABC is not approved by USFDA and not available in USA for sale.
Product Specifications
| Component | Specification | ||
| Balloon Volume (CC) | 30 | 35 | 40 |
| Balloon Diameter(mm) | 14.5 | 15 | 15 |
| Balloon Length(mm) | 225 | 227 | 259 |
| Balloon Catheter Length(mm) | 870 | ||
| Balloon Catheter Usable Length(mm) | 710 | ||
| Catheter Profile(French) | 8 | ||
| Inner Lumen ID (inch) | 0.028 | ||
| Guidewire Compatibility (inch) | 0.025 | ||
| Nominal Pressure (PSI) | 2 | ||
| Maximum Dilation Pressure (PSI) | 5 | ||
| Console Compatibility | Datascope : System 97, 98, 98XT, CS 100, CS 300, CardioSave | ||
| Arrow : ACAT, AutoCAT, AutoCAT 2, AutoCAT 2 Wave, KAAT II | |||
Size Chart
| MYOSIST IAB Catheter kit 8F | 30CC | 35CC | 40CC |
Clinical Data
Product IFU
Note: IFU will be displayed after MDR Certification