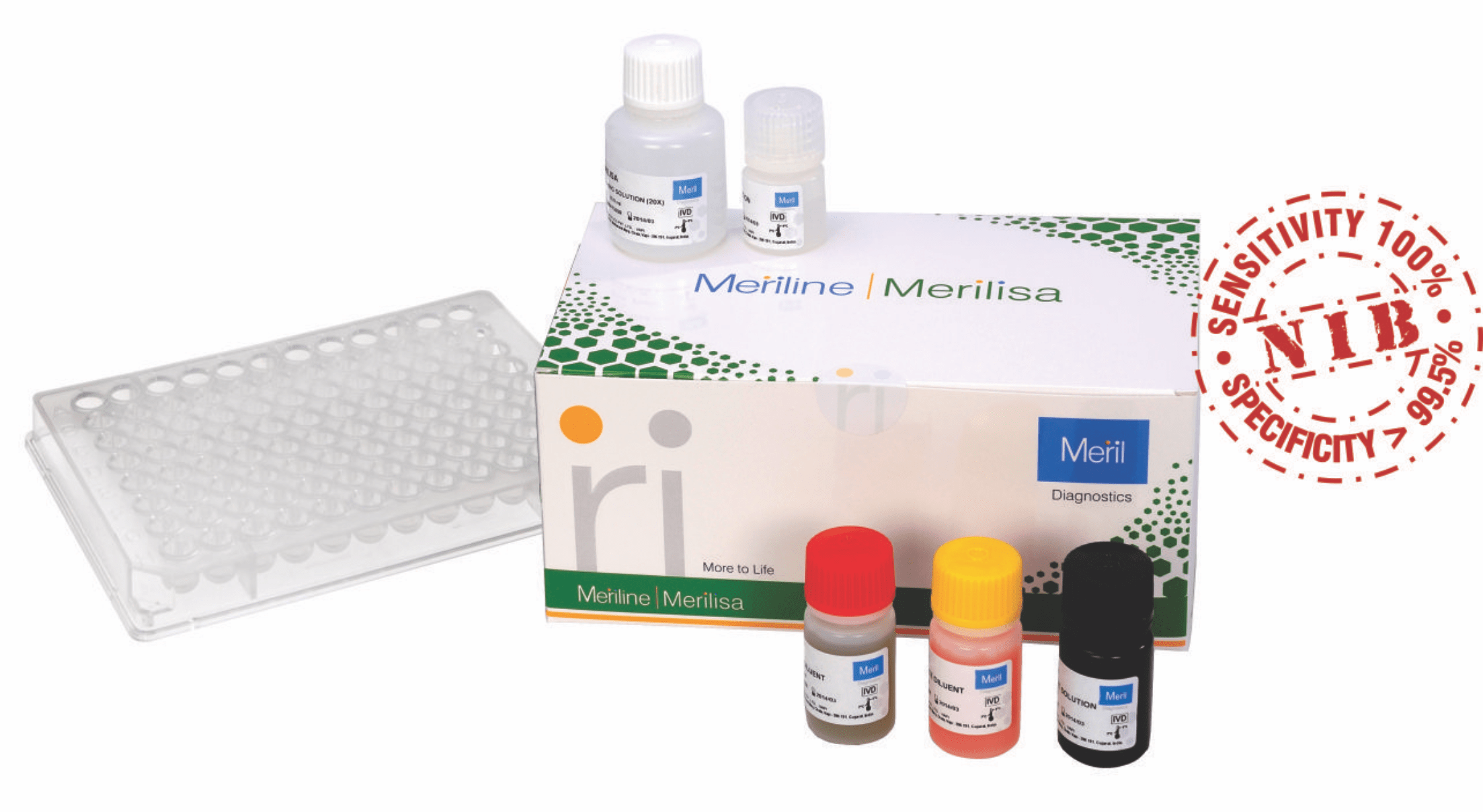
MERILISA HIV 4TH GEN
Merilisa HIV Gen 4 is an enzyme immunoassay for the qualitative determination of HIV p24 antigen and antibodies to Human Immunodeficiency Virus type 1 (HIV-1) and type 2 (HIV-2) in human serum or plasma. Merilisa HIV Gen 4 detects HIV p24 antigen in addition to IgG, IgA and IgM antibodies to the core and envelope glycoproteins of HIV 1-2 by using p24 monoclonal antibodies and recombinant proteins of gp41, gp120 and gp36 representing the immunodominant regions of HIV 1-2.
- Streptavidin biotin technology
- Simultaneous detection of Antibodies and Antigens
- Monoclonal Antibodies for p24 Ag detection
Benefits
01
Significant reduction in window period upto 2 weeks
02
Analytical sensitivity : 10 IU/ml for p24 Ag
03
Separate positive controls for Antigen and Antibody
04
Adaptable on all automated ELISA processors
Indications:
- Simultaneous detection of HIV p24 antigen and antibodies to Human Immunodeficiency virus types 1 (HIV- 1) and 2 (HIV - 2)
Product Specifications
| Intended Use | 4th Generation based immunoassay for qualitative determination of antibodies against HIV 1 and HIV 2 and p24 antigen |
| Principle | Antigen and antibody Sandwich Immunoassay |
| Technique | ELISA |
| Detection | Antibodies to HIV -1 and HIV -2 (IgM, IgG and IgA) and p24 antigen |
| Capture | gp 41 and gp 120 for HIV -1 and monoclonal Anti - p24 ; gp 36 for HIV -2 |
| Monoclonal anti-HIV p24 antibody conjugated to biotin | |
| Conjugate | Mixture of streptavidin and HIV antigen conjugated to HRP |
| Type of Specimen required | Serum or Plasma |
| Total incubation time | 90 minutes |
| Diagnostic Sensitivity | 100% |
| Diagnostic Specificity | > 99.5% |
| Evaluation | National Institute of Biologicals |
Ordering Information
| Product Code | Material Description | Pack Size |
|---|---|---|
| HV4ELI-02 | Merilisa HIV Gen 4 | 96 Wells |
Clinical Data
Product IFU
Note: IFU will be displayed after MDR Certification