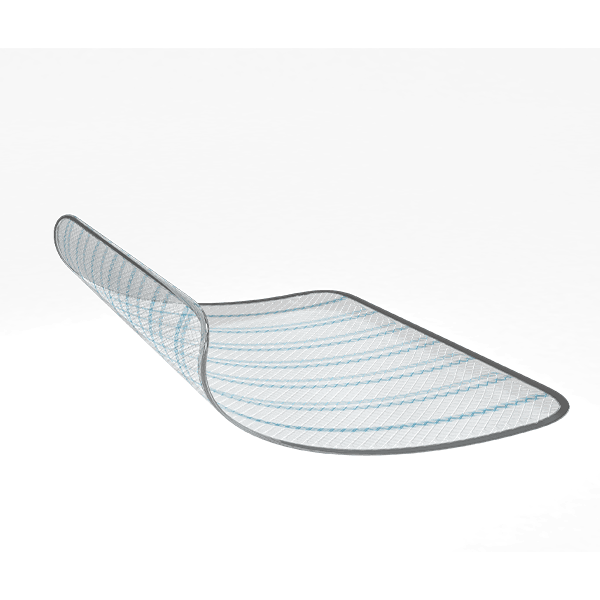
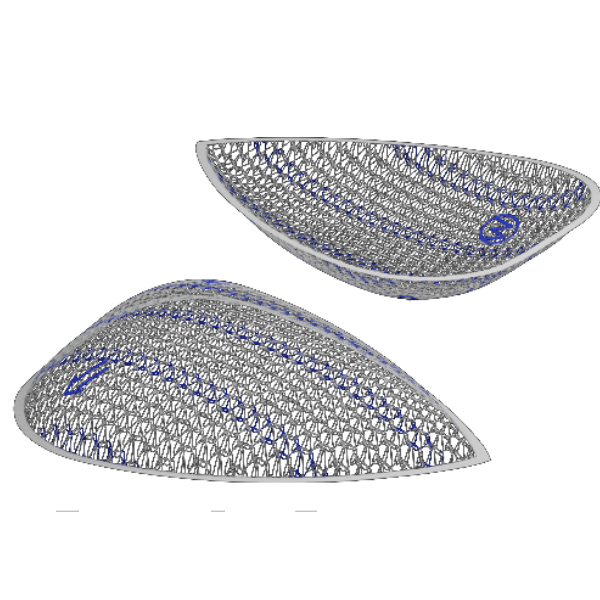
MERINEUM™ Mesh is a sterile, tissue separating dual layered partially absorbable surgical mesh consisting of knitted non absorbable polypropylene mesh and absorbable non-adhesive polylactide-caprolactone film. The polypropylene mesh side of the device allows for tissue in growth and provide additional support to weakened or damaged tissue while the polylactide-caprolactone film acts as anti adhesive layer and physically separates the polypropylene mesh from underlying tissue/organ surfaces during the healing to minimize the risk of unintended tissue attachment to the mesh. The mesh contains the ‘F’ marking on non-adhesive polylactide-caprolactone film which indicates the front side of the mesh. The MERINEUM™ MESH is available in various sizes.
Key Features
Blue lines for Mesh Orientation
IFU
For your convenience, you may select your product at this link to view the IFU.
Indication
MERINEUM™ MESH is indicated to be used for tissue reinforcement in Laparoscopic Ventral Hernia Repair and other fascial surgical intervention procedures.