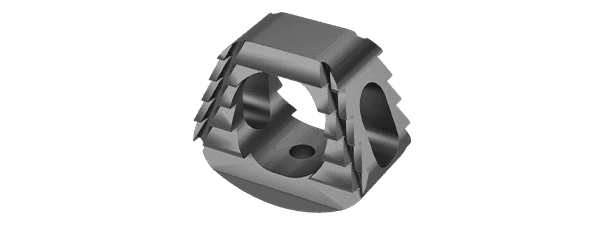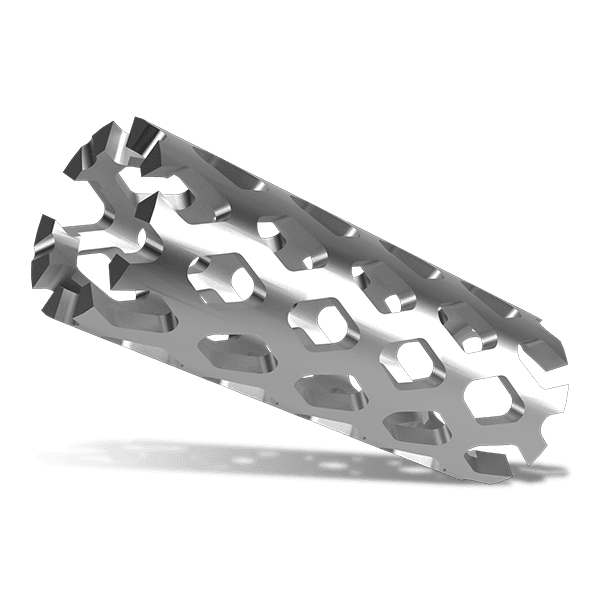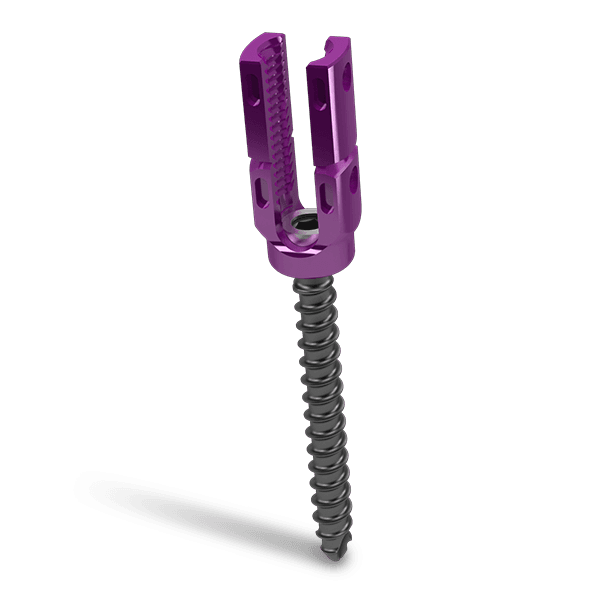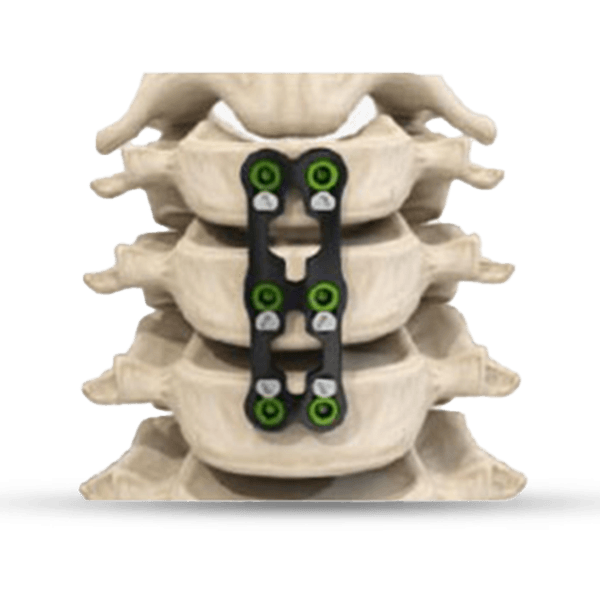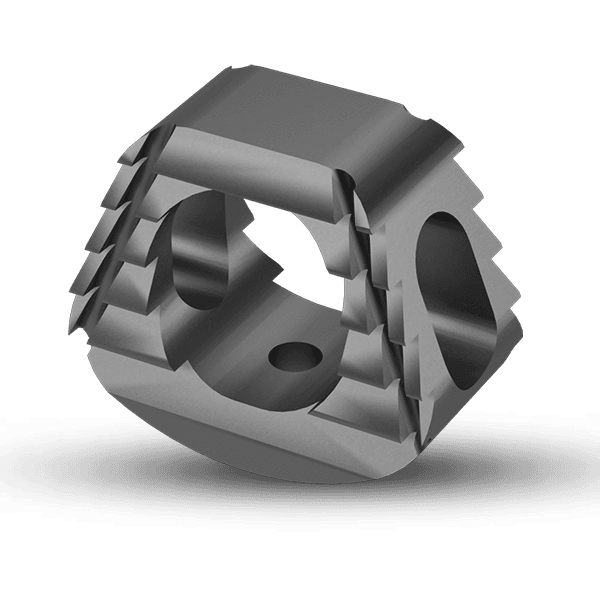
The KEDGE Interbody Fusion System will be offered in various device configurations based on surgical approach and patient anatomy, and consist of:
Cervical interbody fusion device(s), which may be implanted as a single device via an anterior approach.
Key Features
Indication
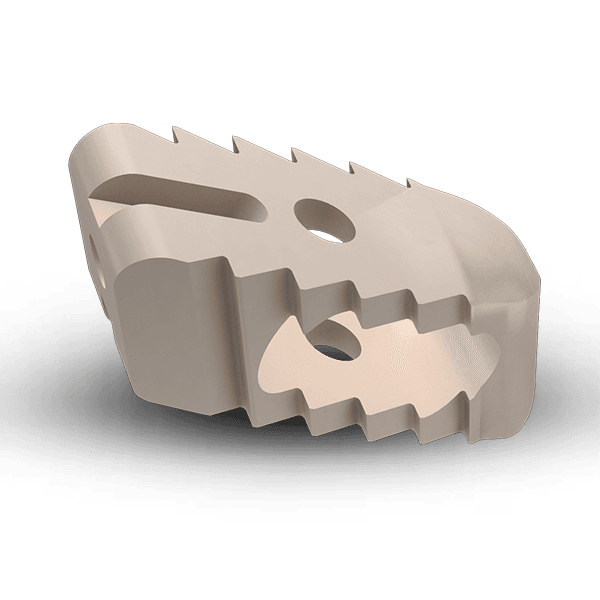
Cervical intervertebral body fusion device, Interbody Fusion System is indicated for intervertebral body fusion in skeletally mature patients with degenerative disc disease (DDD) of the cervical spine with accompanying radicular symptoms at one disc level from the C2-C3 disc to the C7-T1 disc