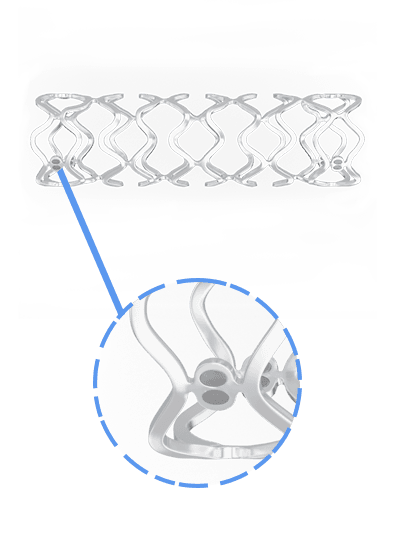
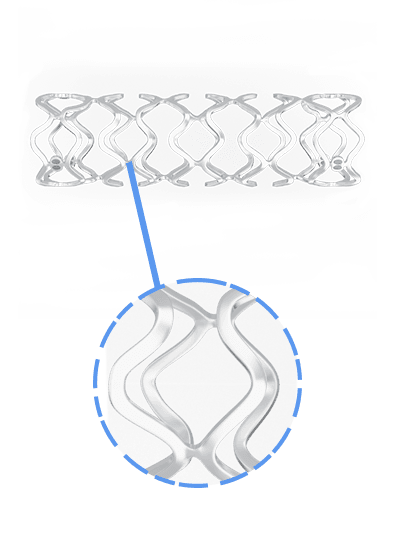
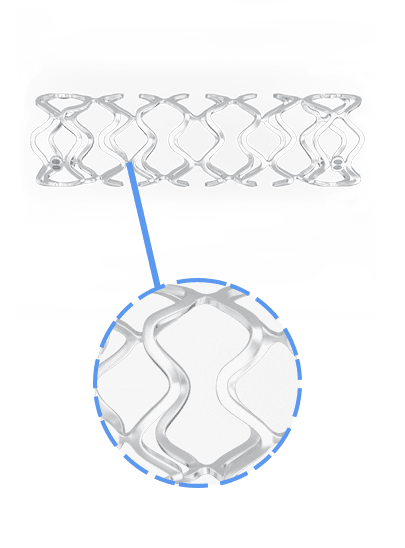
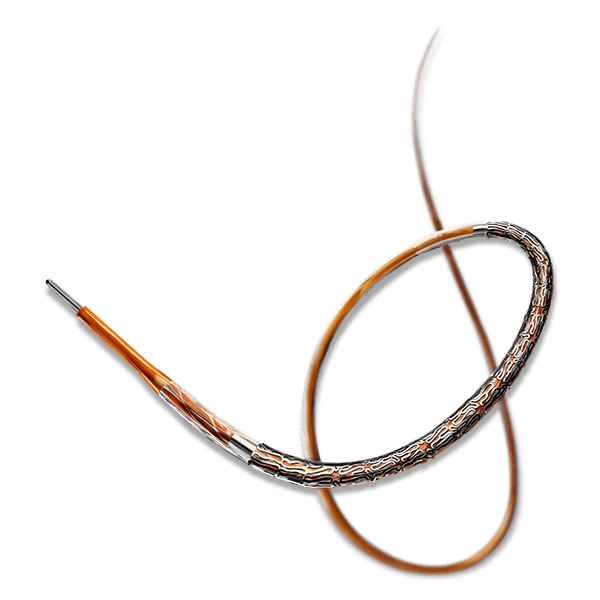Overview of BRS
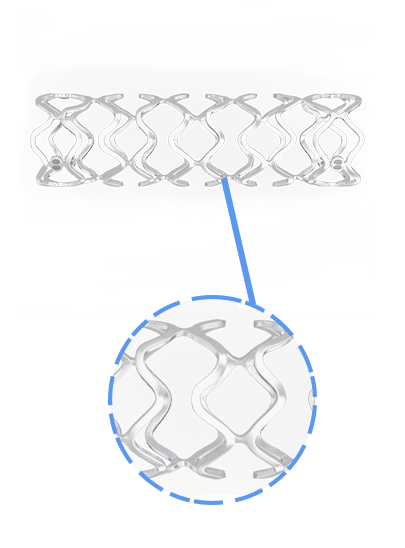
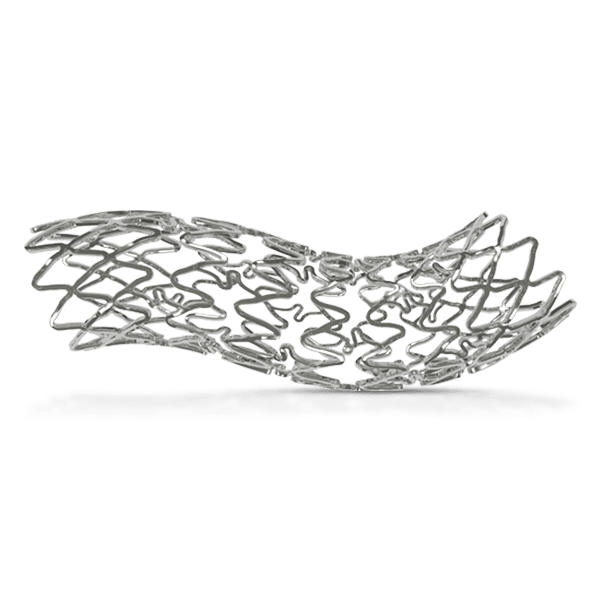
Traditional metallic drug-eluting stents (DES) have been instrumental in restoring blood flow and supporting arterial healing. However, advancements in stent technology have introduced bioresorbable scaffolds (BRS) as a temporary support system. BRS are designed to provide structural support to the treated artery, restoring natural blood flow. Once healing is complete, these scaffolds fully dissolve, leaving the artery in its natural state without any residual foreign material. This innovation allows both physicians and patients greater flexibility for potential future interventions within the same blood vessel, supporting a long-term approach to vascular health.
Key Features
Evolution of PCI
The promise of BRS: Unique Chemistry
MERES100 Angioplasty- Treatment for Coronary Artery Disease