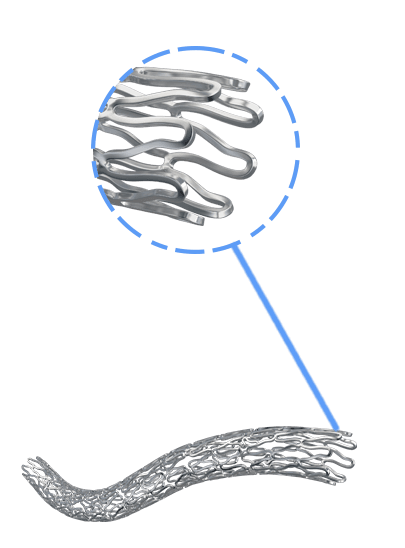
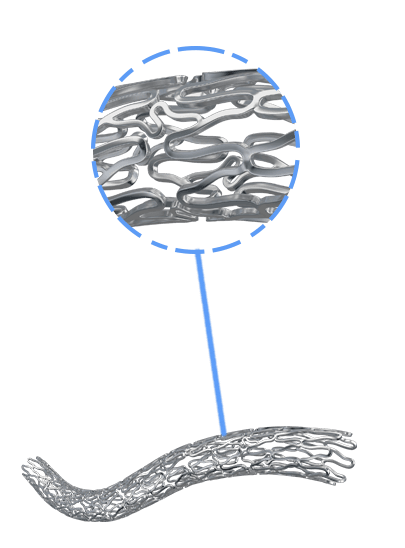
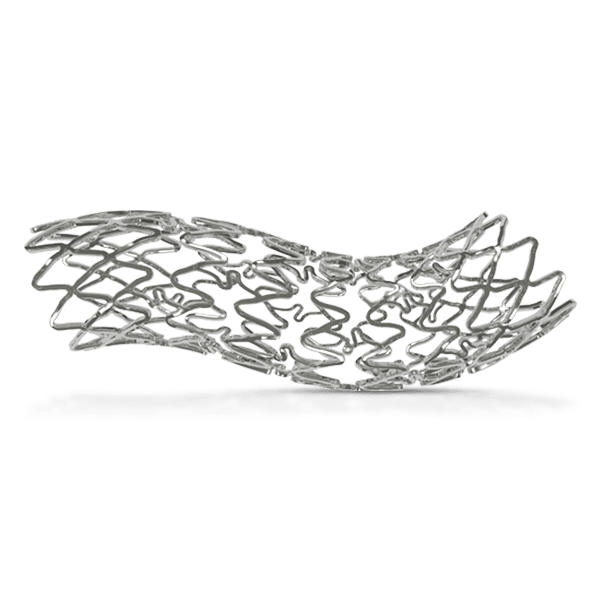
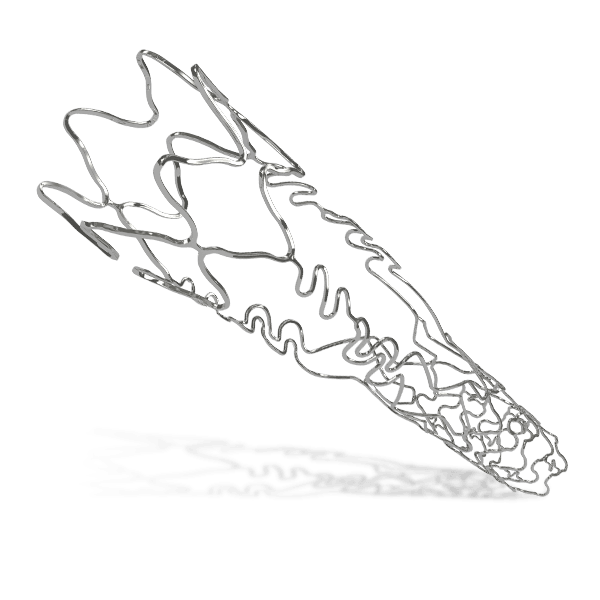
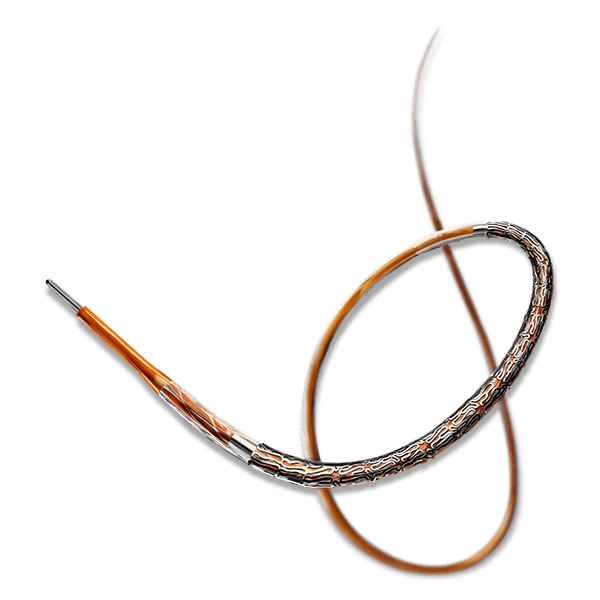BioMime™ Morph brings the proven benefits of BioMime™ in a tapered stent system, allowing the treatment of long, diffused lesions with a single stent—eliminating the need for multiple overlapping stents, which are associated with higher rates of restenosis and revascularization. Designed to taper according to vessel anatomy, BioMime™ Morph helps avoid multiple stent placements, reducing the probability of fracture and restenosis. It is a Sirolimus Eluting Tapered Coronary Stent System with an ultra-thin 65 µm strut thickness, featuring a Novel Hybrid Design with closed cells at both ends and open cells in the middle, enabling Morphology Mediated Expansion for optimal conformability and minimal edge dissections. The BioPoly biodegradable polymer, with a low 2µm thickness, ensures stability and elasticity. Additionally, the stent's design incorporates non-linear S-links and Y-connectors, providing high flexibility and adequate side branch access, while strut width variability ensures optimal radial strength.
Key Features
Highlights
- 0.3% Stent Thrombosis
- 2% Mace at 12 months in Biomime morph multicenter study
- 99.4% freedom from target lesion failure (TLF) at 6 months in morph India study
Indication
Please review the instructions for use prior to using these devices for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.