Delivering Advanced Healthcare Solutions Through Research And Innovation To Improve The Quality Of Human Life
Medical Devices
Advantages of Minimally Invasive Heart Valve Replacement
March 18, 2025

Discover the advantages of minimally invasive heart valve replacement, including faster recovery, reduced trauma, and improved outcomes for patients and doctors.
Medical Devices
The Role of Climate Change in Dengue Spread
March 03, 2025

Learn how climate change contributes to the increasing spread of dengue and discover effective prevention and control measures for this public health issue.
Medical Devices
Patient Outcomes: Traditional vs. Robotic Knee Replacements
February 22, 2025
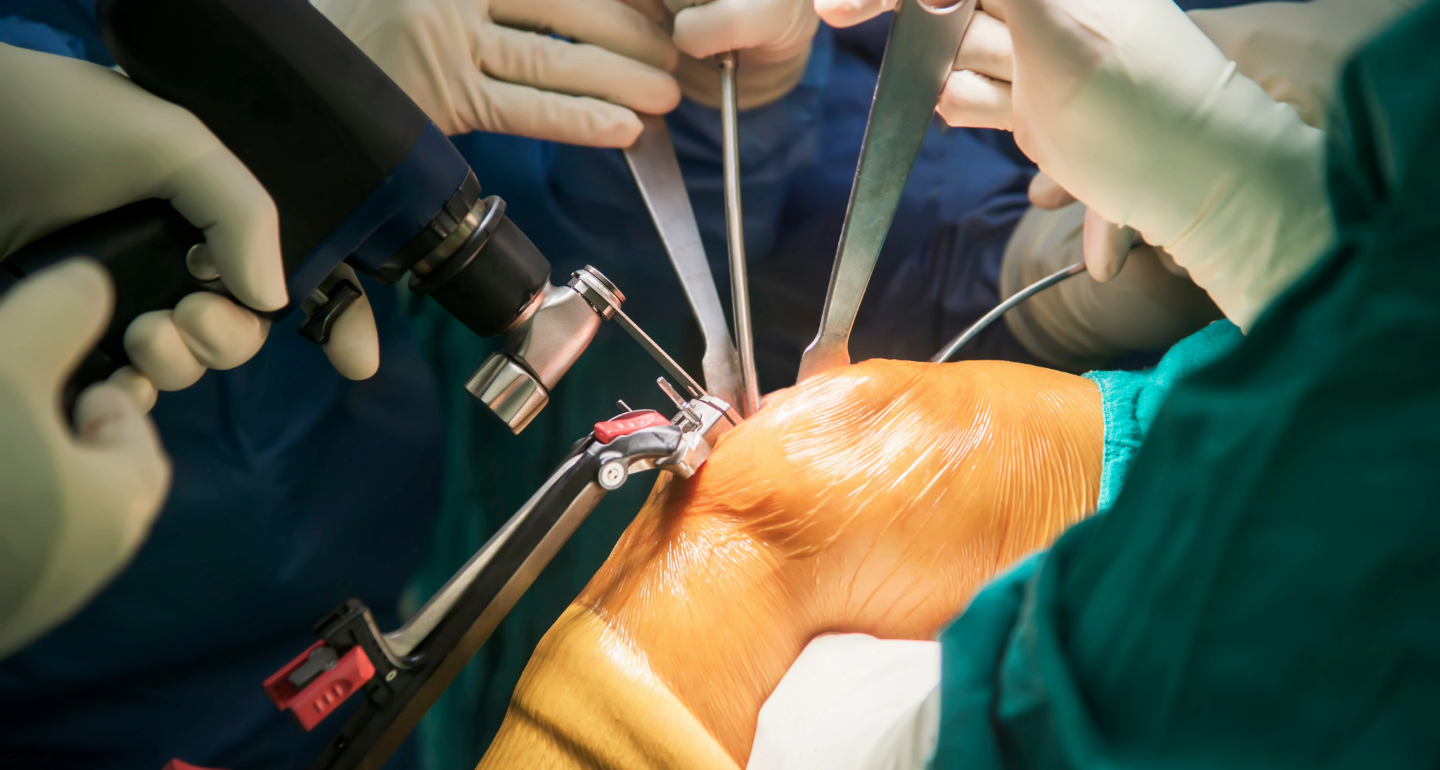
Discover the differences between traditional and robotic knee replacement surgeries. Learn about procedures, benefits, and patient considerations in this detailed guide.